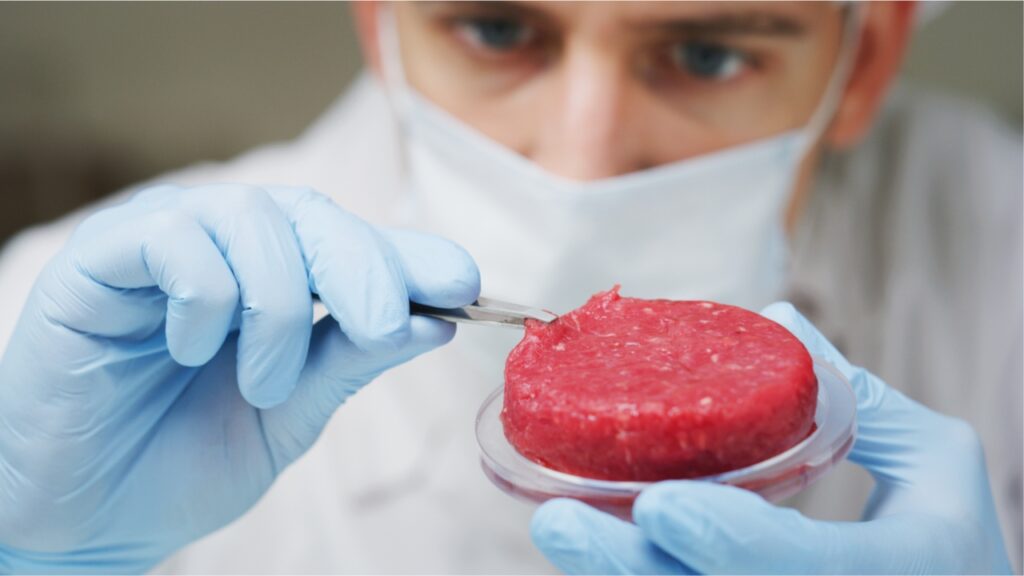
Lab grown meat, or synthetic alternative protein (SAP), is cultivated from animal cells in a tightly controlled process under cleanroom conditions. These sterile, engineered spaces are designed to maintain very low concentrations of airborne particulates and are equipped with filtration systems that remove pollutants such as dust, airborne microbes, and aerosol particles. Workers also wear cleanroom clothing such as coats, gloves, and headgear to prevent contamination from particles on the human body.
Cleanliness, Consistency, and CGMP
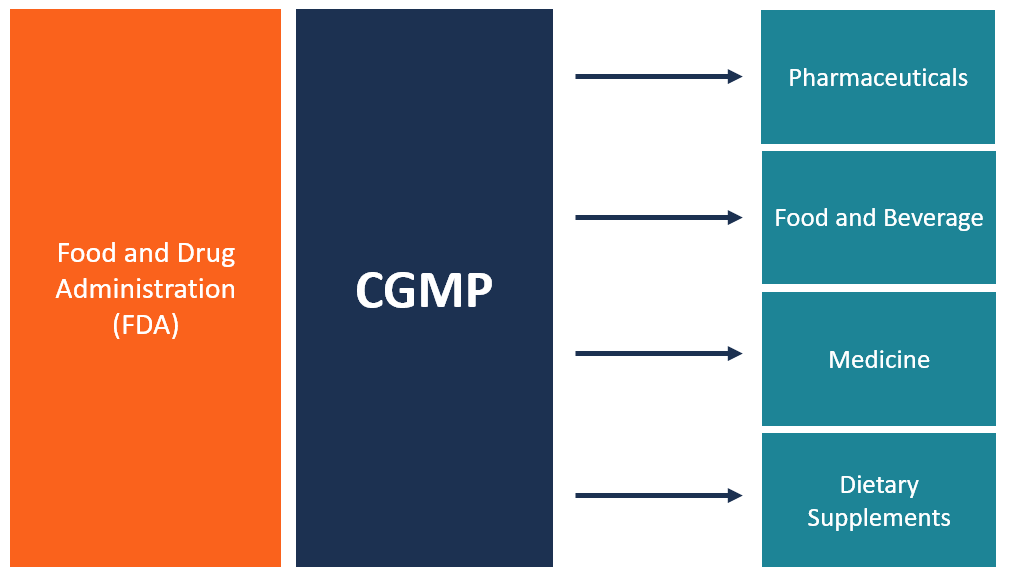
SAP production is sometimes compared to brewing beer, but the level of cleanliness that’s required is more like that of the pharmaceutical industry. As in other laboratory and manufacturing processes, SAP production also requires a high degree of consistency. In the United States, Current Good Manufacturing Practices (CGMP) for food and medicine production apply to SAP and cover equipment, facilities, and controls. Producers also need to clean and maintain equipment properly.
The USDA and FSIS
In the United States, SAP safety is the responsibility of the Food and Drug Administration (FDA) and the Food Safety and Inspection Service (FSIS), which is part of the United States Department of Agriculture (USDA). In 2019, FDA and FSIS established a joint regulatory framework for food made from cultured animal cells. In June 2023, USDA granted its first-ever approval for SAP produced by two companies.
The Global Regulatory Environment
Other countries have their own regulations. In December 2020, the Singapore Food Agency approved cell-cultured chicken, making the Southeast Asian nation the world’s first country to approve an SAP product for sale. The Netherlands, the EU’s largest meat exporter, is the first country in Europe to provide consumer access to SAP under a process governed by the European Union’s Novel Foods Regulation. In Canada, researchers in Alberta have been studying SAP production since at least 2020, the same year in which the Canadian Food Inspection Agency (CFIA) began considering guidelines for simulated meat products.
In our next article, we’ll examine how SAP production begins with stem cells and satellite cells.